What is Ozempic (semaglutide)
Ozempic (semaglutide) is the most recently approved medication in a class of diabetes agents called GLP-1 analogs. Ozempic was approved by the FDA in February 2018 as a weekly injection, in addition to diet and exercise, for the treatment of type 2 diabetes. Like other GLP-1 agonist medications, Ozempic mimics and binds to the body’s own GLP-1 receptor. This results in increased insulin secretion and lower levels of glucagon after eating, slowed stomach emptying, and appetite suppression. Learn more about how GLP-1 agonists work here.
Advertisement
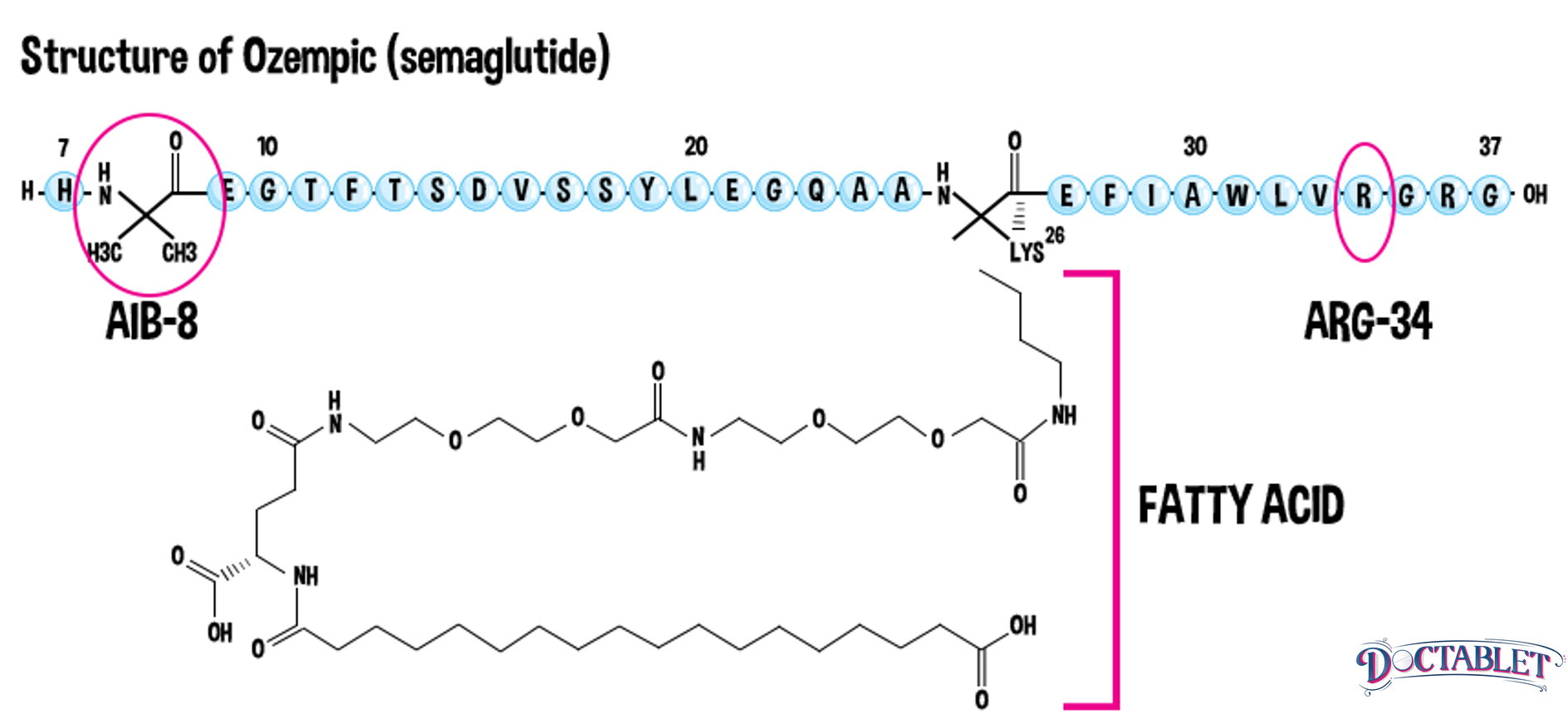
Because patients with type 2 diabetes are both deficient in and resistant to their own GLP-1 hormone, providing GLP-1 as a medication using high pharmacologic doses is considered to be a second-line treatment option for many patients. Over twenty years have passed since the discovery of the first GLP-1 analog, exendin-4 While the original GLP-1 agonist was an amazing advance in diabetes, it only has a 53% structural similarity to the body’s own natural GLP-1 hormone, and a short half-life. In the laboratory, scientists continue to create modifications to the GLP-1 molecule to slow its natural degradation, allowing it to last longer in the body so it can be used as treatment for type 2 diabetes. Ozempic was discovered in 2012 and is structurally very similar (94%) to the human body’s own GLP-1 hormone. It differs by only two substitutions and a special chemical change at a third location. These modifications allow Ozempic (semaglutide) to hang around much longer bound to a protein called albumin, and resist being broken down by DPPIV enzymes whose job it is to deactivate GLP-1. The structural changes made to Ozempic allow it to be given as a once-per-week injectable medication. Now, Ozempic is pulling ahead as the new GLP-1 champion!
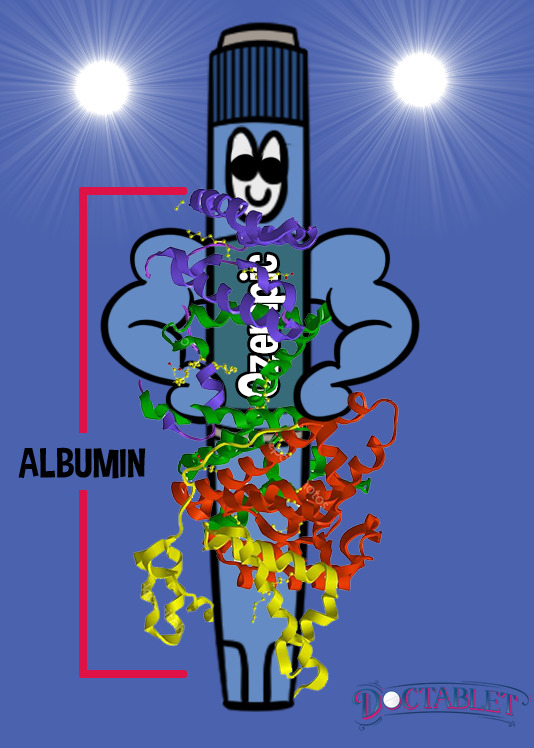
The Ozempic® Pen
Ozempic is currently only available in an injectable, pen form. There are two different pens available: a starting 0.5 mg pen and a second, higher dose of 1.0 mg. It is recommended to use the 0.5 mg pen and start at the 0.25 mg dose once per week for the first four weeks, after which increasing to 0.5 mg once per week is acceptable. Ozempic can be administered any time of day, with or without meals.
What are the side effects of Ozempic?
The most commonly experienced side effects in patients on Ozempic are nausea and vomiting. Diarrhea, abdominal pain, and constipation have also been reported. There are FDA warnings for patients with specific types of thyroid cancer (medullary) and a history of pancreatitis not to take Ozempic.
Ozempic and retinopathy
In a two-year study, Ozempic increased the risk of retinopathy: damage to the small blood vessels in the back of the eye). (3% in patients on Ozempic as compared to 1.8% in patients on placebo). The patients in this study were at high cardiovascular risk, but the potential risk means that all patients with a history of diabetic retinopathy, regardless of whether they have heart or kidney problems, should be monitored if starting treatment with Ozempic (semaglutide). One thought as to why an increased risk of retinopathy was observed is that Ozempic causes substantial improvements in blood sugar control, and this rapid shift in glucose could potentially raise the risk of worsening diabetic-associated eye disease.
The GLP-1 Rumble
With the approval of Ozempic (semaglutide), doctors and their patients now have SIX injectable medications in the GLP-1 class to choose from. Of the six options, three choices are weekly injections that can be used to treat type 2 diabetes. The main differences and comparison of Ozempic to other GLP-1 analogs is outlined below. Ozempic has been tested in a series of important studies referred to by the acronym “SUSTAIN.” These studies have helped doctors understand how Ozempic compares to other medications used for the treatment of type 2 diabetes.
Victoza vs. Ozempic
Many years before the launch of Ozempic, Novo-Nordis released a daily GLP-1 analogue called Victoza (FDA-approved in February 2010). Like Ozempic, Victoza is also 97% similar in structure to the body’s naturally produced GLP-1. Through a chemical process called acylation, Victoza (liraglutide) has a fatty acid attached that allows Victoza to bind more tightly to a protein in the tissue and blood called albumin. This tighter bond prolongs Victoza’s activity in the body and allows it to be injected daily. As compared to Victoza (liraglutide), Ozempic has a three-fold lower attraction for the body’s GLP-1 receptor, but a much longer half-life. This is because it binds to albumin more strongly, allowing it to be injected on a weekly basis. Ozempic (semaglutide) has locked up with Victoza in a smaller, open-label diabetes study, showing slightly better blood sugar control and weight-loss.

The major differences between Ozempic and Victoza in their structure, half-life, and injection frequency are outlined in the table below.
Victoza
Ozempic
Year released
2010
2017
Class
GLP-1 agonist
GLP-1 agonist
Half-life
11-15 hours
1 week
Injection frequency
Once daily
Once weekly
Difference vs. human GLP-1
97% homologous
1 amino acid substitution and acylation of GLP-1-(7-37)
94% homologous
2 amino acid substitutions, acylation of GLP-1-(7-37)
Ozempic vs. Trulicity
Trulicity (dulaglutide) was the second weekly injectable GLP-1 agonist medication approved by the FDA for the treatment of type 2 diabetes. How does Trulicity square up against Ozempic? In a one-on-one, SUSTAIN-7 trial match studying patients already on the medication metformin, Ozempic was found to be superior to Trulicity. After ten weeks, Ozempic hammered Trulicity through the ropes as patients on both the lower and higher doses of Ozempic had better HgbA1c reductions and lost more weight as compared to Trulicity.

Trulicity (dulaglutide)
Ozempic (semaglutide)
Year released
2014
2017
Class
GLP-1 agonist
GLP-1 agonist
Half-life
5 days
1 week
Steady-State
2 to 4 weeks
4 to 5 weeks
Injection frequency
Once weekly
Once weekly
Difference vs. human GLP-1
90% homologous
GLP-1-(7-37) linked to Fc fragment of human IgG4 (https://www.ncbi.nlm.nih.gov/pubmed/21154170)
94% homologous
2 amino acid substitutions, acylation of GLP-1-(7-37)
Ozempic vs. Bydureon (Exenatide Extended Release)
Ozempic had yet another clean finish in the SUSTAIN-3 study when it pinned Bydureon to the mat for a three-count. In a group of over 800 diabetic patients taking oral pills only and studied for over a year, Ozempic (semaglutide) was found to lower A1c values by 1.5% as compared to Bydureon’s 0.9%. In this old-fashioned beatdown, patients on Ozempic also lost more weight on Ozempic as compared to Bydureon (5.6 vs. 1.9 kg).

Bydureon (Exenatide)
Ozempic (semaglutide)
Year released
2012
2017
Class
GLP-1 agonist
GLP-1 agonist
Half-life
2.4 hours (Byetta)
1 week
Injection frequency
Once weekly
Once weekly
Difference vs. human GLP-1
53% homologous
Synthetic Exendin-4
94% homologous
2 amino acid substitutions, acylation of GLP-1-(7-37)
Ozempic vs. Saxenda (Ozempic and weight loss)
Ozempic has not yet been studied as compared to Saxenda. Saxenda is liraglutide at the 3 mg dose, and is currently not indicated by the FDA for the treatment of diabetes. Instead, Saxenda is indicated for overweight patients with complications from their weight or in those patients with obesity. Due to the excellent weight-loss seen in clinical trials for patients taking Ozempic for diabetes, Ozempic is now looking to be a contender in that ring, as well. Ozempic is currently undergoing studies that might help its approval for a weight-loss indication.
Ozempic and heart disease
In the SUSTAIN-6 study , researchers set out to determine the cardiovascular safety of Ozempic in over 3000 patients with type 2 diabetes at high cardiovascular risk. Over two years, there were 26% fewer major heart- and stroke-related events in those patients on Ozempic as compared to the standard of care (placebo). The conclusion of this very important study was that Ozempic has no increase in cardiac or vascular risk. Studies to prove patients have improved outcomes while on Ozempic are currently ongoing.

Ozempic cost
The cost of the Ozempic is approximately $714.
Share this with a patient or friend.
Advertisement

